Master’s thesis research studied the production of the enzyme cyanase in E. coli in a chemostat at varying dilution/specific growth rates. Constructed plasmid containing cyanase cynS gene and Cmr gene coding for chloramphenicol acetyltransferase (CAT). Transformed plasmid into E. coli. Built small chemostat in walk-in environmental chamber and operated at varying dilution rates with and without induction of cyanase expression.
| Unit operation(s): Continuous fermentation |
| Product(s): Recombinant protein |
| Company: University of Minnesota |
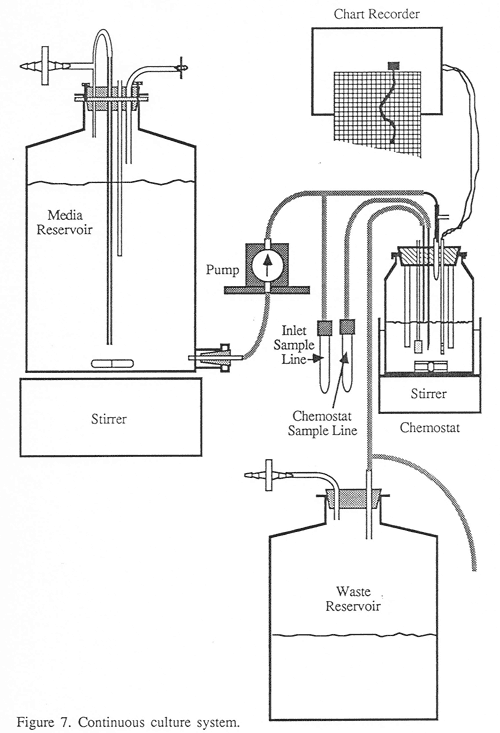
I did my chemical engineering master’s research in the laboratories of Michael Flickinger when he was the director of the University of Minnesota BioTechnology Institute (BTI). I first met Mike when I was studying fuel ethanol production from waste starches at the newly created Natural Resources Research Institute (NRRI) as he was just establishing BTI. Working in Mike’s labs was a tremendous learning experience. We had access to all of the BTI pilot facilities and there were a number of interesting projects going on, including development of an amino acid production process using a thermophilic methylotroph. For that project, we used a silicon tubing volatile organic carbon sensor to monitor the methanol concentration in the fermentor. I later used this same sensor concept in my work developing a m-hydroxyphenylacetylene biotransformation process.
Mike also believed that chemical engineers need to understand the life sciences and life scientists need to understand chemical engineering to work together in the field of biotechnology. To promote this, his laboratory included undergraduates, graduate students and postdocs from microbiology, biochemistry, molecular biology and chemical engineering. This was a perfect environment for me and I learned a lot from the life scientists in the group.
For my thesis research, they started by teaching me how to work with recombinant DNA and construct my own plasmids and transform them into E. coli. Once I had the strains created, I built a small chemostat in a walk-in environmental chamber in the lab. In those days, anyone doing fermentation research had to build their own galvanic dissolved oxygen probes using lead and silver wires, glass tubing and a soldering iron. Once I had the strain and the chemostat, I set about running it at a variety of dilution rates. The goal of the project was to understand protein expression in bacteria at slow growth rates. This meant low dilution rates and periods of days for the cultures to line out at each set of conditions. With only a single chemostat, this required considerable patience and good sterile technique. The chemostat was regularly sampled and assayed for cyanase activity and the cells examined under a microscope.
One of the most interesting observations was that at very low growth rates, the E. coli cells became filamentous. As CAT inactivates chloramphenicol, at the low growth rates, I found I needed a very high chloramphenicol concentration in the feed medium to stabilize the plasmid. We never determined if it was the slow growth rates alone or the presence of chloramphenicol or some other factor that induced this filamentous cell formation.
More Reading
Flickinger, M. C., & Rouse, M. P. (1993). Sustaining protein synthesis in the absence of rapid cell division: An investigation of plasmid‐encoded protein expression in Escherichia coli during very slow growth. Biotechnology progress, 9(6), 555-572.